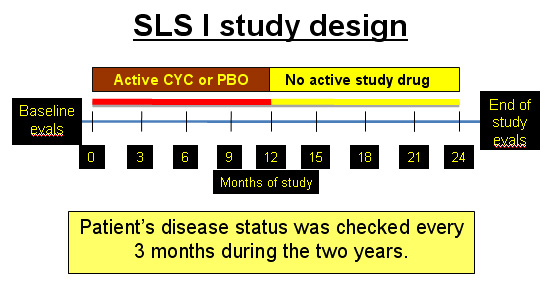
Scleroderma Lung Study I (SLS I) was a clinical research study that compared the efficacy and toxicity of daily oral cyclophosphamide (CYC, also called CytoxanTM) with a daily placebo pill for the treatment of scleroderma-related lung fibrosis. The duration of the study was for 1 year and the patients were followed for an additional year without treatment as outlined in the figure below.
The main findings from the SLS I study are summarized below (please see the section on “Our Publications” for complete scientific details):
A. At the end of 1 year treatment with CYC was:
1. Better than a placebo pill in stabilizing the lung function.
2. Better than a placebo pill in improving symptoms of shortness of breath.
3. Better than a placebo pill in improving quality of life and physical function.
4. Better than a placebo pill in improving skin thickness in patients with diffuse scleroderma.
5. Safe when side effects were closely monitored
B. During additional follow up year (year #2)
1. The effect of CYC on lung function lasted for an additional 6
months but was no different than a placebo pill at the end of 2
years.
2. The effect of CYC on symptoms of shortness of breath continued
at the end of 2 years.
3. The effect of CYC on physical function lasted for an additional 6
months after stopping the drug but was no different than a
placebo pill at the end of 2 years.
4. The effect of CYC on skin thickness persisted at the end of 2
years.
5. There were no safety concerns with CYC at the end of 2 years.
In summary, the SLS I study showed that 1 year of oral CYC was effective in improving lung function, symptoms of shortness of breath, and quality of life but the effect on lung function and quality of life only lasted for another 6 months after CYC was stopped. CYC caused few serious side effects for most study patients when the drug was carefully monitored according to the protocol. SLS II builds on these findings and will compare CYC with MMF to learn which one provides the best outcomes after two years.